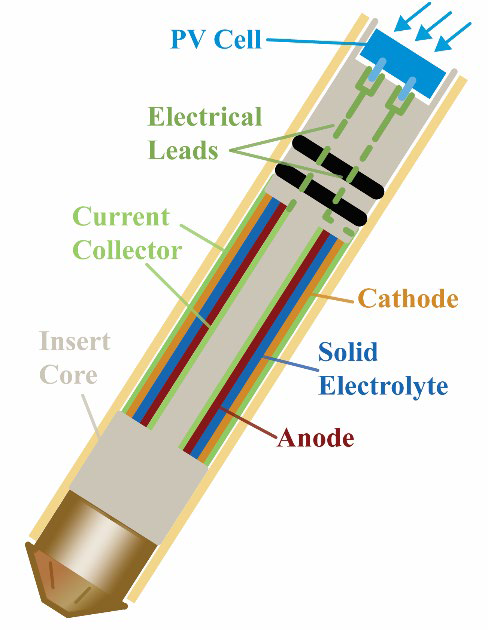
Solid-state batteries are a safer and higher energy density alternative to current liquid electrolyte batteries. Their performance has been hindered by the solid-solid interface (SSI) that forms between the solid electrolyte and electrodes. Insight into the SSI chemistry is exceptionally difficult using standard interrogative techniques as the SSI is nanometers thick, highly disordered, and buried deep within the electrochemical cell precluding any information on the dynamics of formation. Recent studies on solid-state batteries have focused on ex-situ characterization, looking at the changes induced after cycling either in the charged/discharged state however these techniques do not offer any insight into the complex kinetics and metastable phase transformations occurring under bias at the SSI. Understanding the atomic mechanisms occurring at the biased SSI is critical to a breakthrough in the development and enhancement of solid-state battery performance.
Probing the SSI during charging and discharging requires a non-destructive operando experimental technique sensitive to the small quantity of the SSI and capable of detecting simultaneously structure and dynamics, all within a fully operational cell. Solid-state nuclear magnetic resonance (NMR) spectroscopy is uniquely suited to address these challenges as it is sensitive to local structure and dynamics and the signal arises from the entirety of the sample volume, making buried interfaces observable. A complication for solid state NMR experiments is the requirement of spinning the sample at high speeds to enhance spectral resolution. The sample spinning requirement creates many technical challenges due to the metallic components generating eddy currents and further requires novel ways of charging the battery wirelessly for the operando capability. Although operando NMR has been developed for liquid electrolyte batteries, these methods cannot be extended to solid-state samples as there are numerous broadening mechanisms for solids that prevent any signal from being adequately measured. LLNL has developed technology to surpass these limitations and provide operando capabilities for solid-state magic angle spinning (MAS) NMR.
NMR provides information on specific elements like Li, can easily observe disordered and amorphous phases, and can obtain signal from the whole battery volume so buried features like interfaces are observable. Ex-situ MAS NMR offers complimentary information however lacks the ability to observe dynamics under a bias, as such misses key information regarding the mechanisms of ion dynamics related to battery performance. The operando MAS NMR technique fills the previous experimental gap within solid-state batteries for investigating degradation mechanisms and evolving chemistry of the internal components and their dynamics.
There are prominent technical challenges arising from spinning a battery on the order of kilohertz as required by magic angle spinning in order to obtain spectral resolution that are addressed and enable operando solid-state NMR. The operando NMR measurement allows for continuous monitoring of the battery components and of potential metastable states that may exist during charge cycling. Outside of monitoring the changes of battery components in an operando measurement, this technology enables the user to perform other advanced solid-state NMR experiments while applying an electrical bias thus enabling characteristic information of the materials to be acquired. It should be noted, implementation of this technology requires a NMR spectrometer and integration into a pre-existing NMR probe.
Operando solid-state NMR for batteries fills a critical experimental void by providing direct access to the complex kinetics occurring at the solid-solid interface under bias and allows for monitoring the extent of chemical reactions and their species within the battery during operation. This technology is wholly novel and adds unique capability to solid-state NMR spectroscopy as an experimental platform for deriving information of materials and devices. Solid-state NMR enables advanced experiments and information that is inaccessible by solution-state NMR and other experimental techniques:
- identification of metastable phases that are only present while the battery is charging or discharging, these would not be observable in ex-situ measurements and have important implications to macroscopic battery performance.
- structural information related to short and intermediate range order such as chemical shift anisotropy, dipolar and quadrupolar coupling constants and element specific dynamics via chemical exchange and spin diffusion.
- dynamics of select ions can be isolated whereas dynamics determined from impedance spectroscopy considers the contributions of all charge carrying species.
- changes to ion dynamics under an electrical bias can be directly measured and the source leading to charge transfer resistances can be identified.
As the technology enables the user to perform solid-state NMR experiments on materials or devices under an electrical bias it offers unique atomic level information on material properties. Specifically, it currently has relevance to the study of electrochemical devices like batteries. The information from NMR experiments provides access into underlying mechanisms influencing battery performance. Information regarding the chemical identity of solid-state battery components including elusive interfacial phases and their dynamics are available and can be monitored before, after, and during charging and discharging. This sort of information is vital to research and development of new solid-state battery technologies, so battery focused research groups in academia, government, and industrial sectors are most interested in this technology.
A working prototype of the rotor insert is being developed concurrently with integration of the system into a commercial NMR probe. Previous testing has identified appropriate conditions for charging a solid-state battery. A record of invention for this technology has been filed.