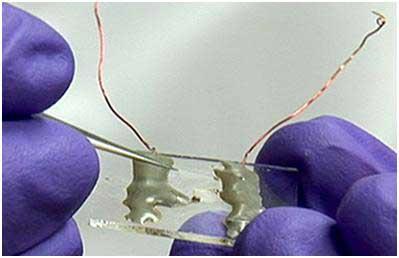
Existing nanosensor technologies depend on an external power source (typically a battery) to operate. Lawrence Livermore National Laboratory has developed a superior alternative: the first batteryless sensors that harvest environmental energy supplies using one-dimensional semiconducting nanowire arrays. The commercial applications for this technology include readily deployable self-powered chemical sensors for military, industrial, medical, and environmental applications. By freeing the nanosensor from a tethered power supply, LLNL scientists have provided the new standard for portable chemical detectors.
Chemical and biological sensors based on nanowire or nanotube technologies exhibit observable ultrasensitive detection limits due to their unusually large surface-to-volume architecture. This suggests that nanosensors can provide a distinct advantage over conventional designs. This advantage is further enhanced when the nanosensor can harvest its meager power requirements from the surrounding environment. A self-powering or "batteryless" device can made small enough to serve in unique situations ranging from military to medical applications. LLNL researchers successfully fabricated two prototype platforms for batteryless chemical detectors using one-dimensional semiconductor nanowires.
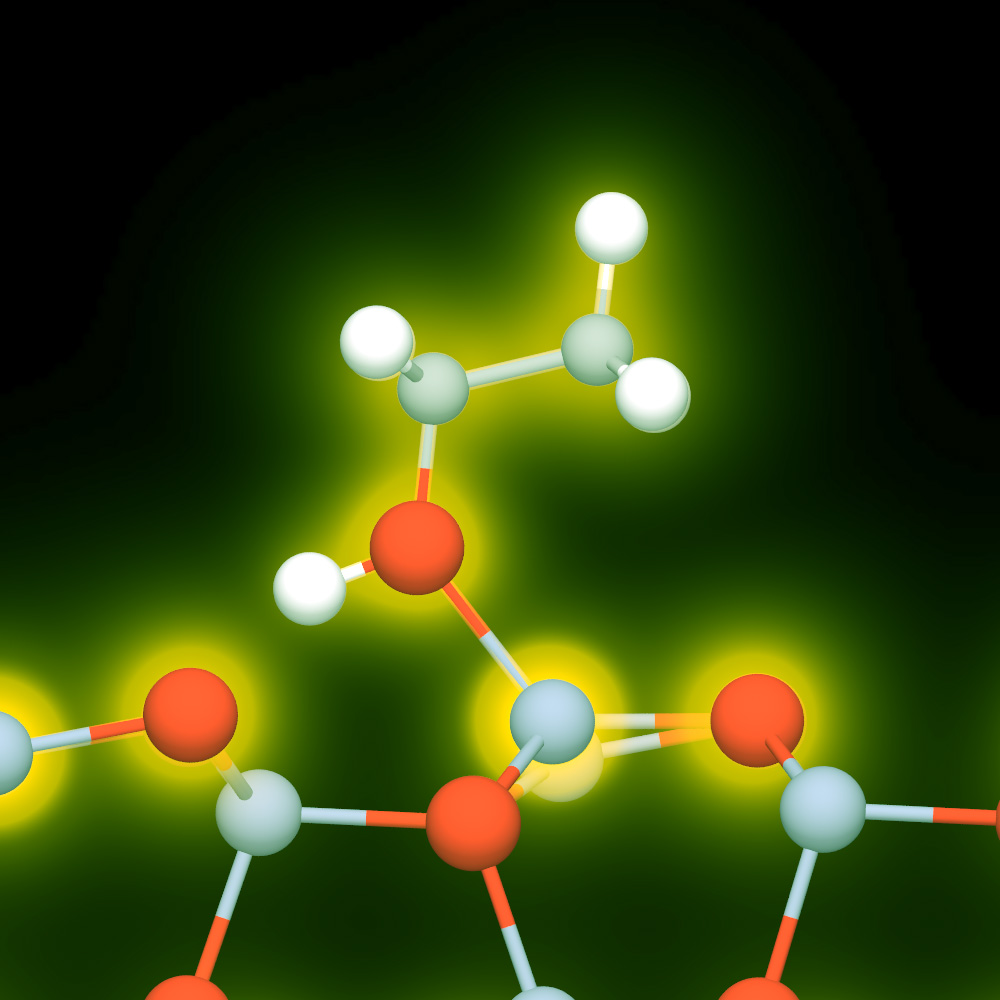
The best currently available nanogenerators can capture, convert, store, and use the energy inherent in piezoelectrics (mechanical/vibration energy), thermoelectrics (heat energy), and photovoltaics (solar and other light energy). The LLNL research team believes that matrix-assisted energy conversion is the next step in eliminating the need for batteries and other external power sources. In this system, polar organic molecules interact with partially exposed nanowires to form a nanoconverter.
This self-powered platform relies on the response of a polymeric film to drive the piezoelectric effect in a nanowire array. The hybrid organic/inorganic platform begins with a nanowire array embedded in an environmentally responsive organic polymer (PVC). This matrix sits atop a support substrate. Both ZnO and boron-doped Si nanowires have proved to be excellent sensor materials. Two sensing platforms were therefore created, based on these two materials. The working principle of the nanosensor on each platform relies on the partial exposure of semiconducting nanowires to target chemical species and a non-ohmic contact that is necessary for the nanosensors to function.
- Converts chemical energy into electrical energy
- External power source (such as a battery) is eliminated, reducing bulk and weight
- Nanosensor responds selectively to a variety of organic molecules, such as ethanol, etc.
- Freedom from thermodynamic equilibrium variables yields response times faster than 1 second
- Heat from the surrounding environment can be harvested to power the detector
- Counterterrorism contraband sensor programs
- Ultrasensitive detection of low-concentration gas leaks (pipeline protection)
- Military, particularly on the battlefield (chemical and biological weapons)
- Wireless sensor networks that monitor the structural integrity of infrastructure such as bridges and dams, and the soundness of airplanes and buses
- Medical control devices such as pacemakers, left-ventricular-assistance units, Insulin control systems, and other monitoring devices
- Potential biological applications, by exploiting the tangible dipole moment found in most organic molecules present in living systems
- Piezoelectric voltage and current outputs powering cell phones, including smart phones
Patent applications have been filed. Prototype is in use at LLNL.
11/09/2010 online at http://pubs.acs.org/NanoLett and 01/04/2011 in Nano Letters
11/30/2010 online at the wileyonlinelibrary.com and 01/04/2011 in Advanced Materials