FLC Regional and National Awards
Scientific researchers, technology transfer professionals, entrepreneurs and visionary business professionals create the stories of technology commercialization success. Along the way to success awards are won for technology transfer efforts. Federal Laboratory Consortium for Technology Transfer (FLC) awards recognize the work of scientific innovation as well as the work of technology transfer professionals that push the research into the private sector.
The ATOM Consortium was founded in 2017 by LLNL; the Frederick National Laboratory for Cancer Research; the University of California, San Francisco (UCSF); and pharmaceutical company GSK (formerly GlaxoSmithKline). It is a multi-institutional computationally-driven initiative to develop a flexible platform for predictive antibody design and to increase pandemic readiness by
accelerating discovery and development of biologics-based medical countermeasures.
LLNL: Jim Brase, Jessica Mauvais, Stewart He, Kevin McLoughlin; FNL: Eric Stahlberg, Justin Overhulse, Naomi Ohashi, Pinyi Lu, Sean Black; UCSF: Amanda Paulson, Rebecca Lein
LLNL's nanolipoprotein particles won the FLC 2024 Excellence in Technology Transfer Award.
This April, LLNL biologist Nicholas Fischer and Yash Vaishnav, a business development executive in the Lab’s Innovation and Partnerships Office (IPO) have garnered a Federal Laboratory Consortium (FLC) technology transfer award and honored during the FLC national meeting in Dallas, Texas.
The FLC award recognizes a LLNL biomedical technology called nanolipoprotein particles (NLPs) that can deliver vaccines and drugs inside the cells of the human body. The advance was licensed to Ann Arbor, Michigan-based EVOQ Therapeutics in 2017. EVOQ Therapeutics has been able to use the NLPs for lymph node-targeted delivery of specific antigens for autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, celiac disease and others.
Yash Vaishnav, Business Development Executive, LLNL Innovation and Partnerships Office
Nicholas Fischer, co-inventor and biologist, LLNL
David Giljohann, CEO, EVOQ Therapeutics
William Brinkerhoff, Co-founder and Board Chair (former CEO), EVOQ Therapeutics
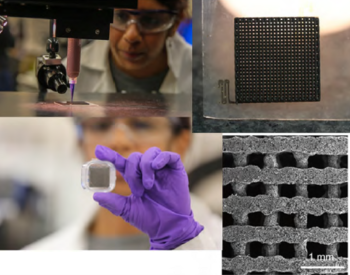
LLNL's Energy Inks won the FLC 2023 Best in Far West Region Award.
Energy Inks offer optimized functional properties to realize next-generation, high-performance 3D-printed devices for energy storage, catalysis, filtration, sensors, and more. By utilizing the design flexibility of direct ink writing, Energy Inks allow customizable battery and supercapacitor components to be 3D-printed faster and at lower cost while exhibiting higher performance than competitive technologies.
LLNL: Marcus Worsley and Swetha Chandrasekaran; MilliporeSigma: Adam Raw and Monica Jung de Andrade | Collaborative support- UC Santa Cruz: Yat Li and Dun Lin; LLNL: Patrick Campbell, Maira Ceron-Hernandez, Alyssa Troksa, Josh Kuntz, Wyatt Du Frane and Genaro Mempin (LLNL Business Development Executive).
Early detection, whether used against bioterrorism or in support of disease prevention, can make the difference between life and death. A team of Lawrence Livermore National Laboratory (LLNL) researchers developed the Droplet Digital™ PCR technology (ddPCR) technology, a subset of digital PCR technologies, that offers advantages over other PCR technologies including cost-effectiveness; ease of use and integration into clinical research and life science workflows; increased signal-to-noise ratio; simplified quantification; and unrivaled precision, accuracy and sensitivity. Licensing the ddPCR technology – first to startups for development and introduction to the market, then to Bio-Rad Laboratories, an international leader in products for the life science research and clinical diagnostic markets – has enabled ddPCR’s commercial use by international researchers and medical professionals.
Yash Vaishnav, Business Development Executive, LLNL Innovation and Partnerships Office
Josh Shinoff, VP Business Development, Life Sciences Group & Digital Biology Group, Bio-Rad Laboratories
As the COVID-19 pandemic revealed a nationwide shortage of ventilators, Lawrence Livermore National Laboratory (LLNL) immediately began designing a durable, portable mechanical ventilator to help fill the gap. To avoid impacts to an already-strained supply chain, LLNL staff set an additional goal of using readily available parts not required by commercial ventilator manufacturers. In just over three months, LLNL and industry partner BioMedInnovations, LLC (BMI) designed, produced, and tested an easily reproducible prototype for a portable, life-saving ventilator and partnered with manufacturing facilities to release SuppleVent™ into the marketplace.
Jack Kotovsky – Principal Investigator; Patrick Dempsey, Director of Strategic Partnerships, Engineering Directorate; Gokhan Yildiz, Principal Engineer; Brent Johnson, Design Engineer; Wayne Lucado, Supply Chain, Design, Testing, Manufacturing and Assembly Engineer; Genaro Mempin, Business Development Executive; Alicera Aubel, Agreements Specialist.
LLNL Design Team: David Soscia, Allison Yorita, Jeremy Gleick, Michael Triplett, Phil Paul, Doug Modlin, Austin Nye, Patrick Scholl, Matt Pharr, Michael Triplett, Ken Enstrom, Ian Ladner, Aaron Sperry, Greg Norton, Victor Vargas, Jack Dean, Brian Wihl, Steven Guzorek, Ian Ladner, Dan Manha, Jacob Trueblood, Shaine Athey.
BioMedInnovations, LLC
Dr. Sherif Gabriel, former BMI CEO; Carrie DiMarzio, BMI CEO.
Equilibar
Jeff Jennings, Founder/President/Mechanical Engineer
A collaboration between Lawrence Livermore National Laboratory (LLNL) and Argon Electronics has led to the development and commercialization of the Radiation Field Training Simulator (RaFTS) technology, an ultra-realistic radiation simulator. RaFTS connects directly to radiation detection instruments to provide a signal indistinguishable from a real radiation source so training in a scenario of interest can take place without the risk of radiation exposure.
With RaFTS, law enforcement, military, and other teams on the front line now have the capability to respond to realistic data using their employer-issued devices in their own jurisdictions without the high cost and risk of transporting and storing radioactive materials. As a result, RaFTS facilitates more sophisticated training scenarios and expands the locations where training can be conducted—all to better prepare first responders to protect the nation and the world from nuclear terrorism and accidents. The patented technology has also earned a 2017 R&D 100 award.
Greg White, LLNL current project leader and technology co-inventor; Steven Kreek, LLNL former project leader and developer; Josh Oakgrove, LLNL, software developer; Dan Bowers, LLNL electrical engineer; Phil Dunn, Argon Electronics engineer; Steven Pike, Argon Electronics CEO, represented Argon Electronics throughout the technology transfer; and Annemarie Meike, LLNL Business Development Executive, negotiated agreements and partnerships with Argon Electronics.
For decades, microcapsule research and development has offered the potential of many new markets and applications, and this market need is growing since the discovery of monodisperse capsule fabrication via microfluidics – but commercialization has been hampered by needed improvements in production methods.
Now LLNL scientists and engineers, in collaboration with Purdue University researchers, have developed the In-air Drop Encapsulation Apparatus (IDEA), which creates microcapsules of consistent size and composition at a rate 100 times faster than current microfluidic-based, single dispersal capsule production techniques, and up to 1,000 times faster when incorporated with a multi-nozzle design. Because capsules can be produced in air instead of solely in liquid, the post-process time and material waste can be reduced by up to 99 percent.
The team receive an “outstanding technology development” award for the FLC’s Far West/Mid-Continent region.
The Lab IDEA team is led by materials engineer Congwang Ye and includes materials engineers Kevin Paulsen, Will Smith, Caitlyn Cook, Eric Duoss, Elaine Lee and Ashley Hall; mechanical engineers Julie Mancini and Kenneth Enstrom; materials chemist Sarah Baker; materials scientist Joshua Kuntz; polymer chemist James Oakdale; environmental engineer Joshuah Stolaroff; chemical engineers Andrew Pascall and Marcus Worsley; and Roger Aines, chief scientist of the Energy Program.
Annemarie Meike is the business development executive (BDE) in the Innovation and Partnerships Office (IPO) who handles the IDEA technology.
Researchers from LLNL, Santa Clara-based Shape Memory Medical Inc. and Texas A&M University received an FLC regional award for outstanding commercialization success for their IMPEDE Embolization Plug, a medical device that prevents continued blood flow to diseased vessels.
IMPEDE, which has been cleared by the Food and Drug Administration for use in the U.S. and has been cleared for use in Europe. IMPEDE reduces the blood flow through diseased or damaged vessels that can occur in abdominal aortic aneurysm endoleaks, gastric and esophageal varices or blunt trauma.
To date, more than 250 patients have been successfully treated worldwide with IMPEDE for conditions such as deformed arteries, tumor resections (where blood flow has been blocked to tumors) and pelvic congestion syndrome, with no reported adverse effects.
LLNL employees who have worked on the project include polymer scientist Tom Wilson, biomedical engineer Jennifer Rodriguez, applied optical physicist Ward Small and computational fluid dynamics engineer Jason Ortega.
As the COVID-19 pandemic surged and concern emerged over a potential nationwide shortage of ventilators, LLNL researchers began designing a durable, portable mechanical ventilator to help fill the gap.
A group of approximately 20 engineers and scientists began prototyping a ventilator that could be made from non-traditional parts, preventing further stress on the already-strained supply chain.
In just over three months, LLNL and industry partner BioMedInnovations, LLC (BMI) of North Carolina designed, produced and tested an easily reproducible design prototype while partnering with manufacturing facilities and gaining Food and Drug Administration (FDA) authorization for the device’s emergency use.
This collaboration was largely done remotely, with scientists, engineers and medical experts contributing from home offices, in many cases, due to shelter-in-place orders.
This work netted an “outstanding partnership” award for LLNL and BMI.
The LLNL ventilator effort is led by mechanical engineer Jack Kotovsky and includes mechanical engineers, Austin Nye, Patrick Scholl, Matt Pharr, Ken Enstrom, Ian Ladner and Dan Manha; mechanical technologists Aaron Sperry, Greg Norton and Victor Vargas; electrical engineers Doug Modlin, Jack Dean and Brian Wihl; physicists Jacob Trueblood and Phil Paul; biomedical engineers David Soscia, Michael Triplett and Jeremy Gleick; chemical engineer Allison Yorita; precision engineer Steven Guzorek; administrator Shaine Athey; and Patrick Dempsey, the director of strategic partnerships and communications for Engineering.
Genaro Mempin is the IPO BDE who has handled the technology transfer work, including a CRADA, for the ventilator project, with assistance from Alicera Aubel, an IPO agreements specialist.