Electrochemical measurements in aqueous solutions are typically measured against a well-characterized and widely used reference electrode (RE), such as silver/silver chloride (Ag/AgCl), saturated calomel electrode (SCE), or reversible hydrogen electrode (RHE). Ag/AgCl reference electrodes can be used in some ionic liquids, however, the high solubility of AgCl in some ionic liquids greatly limits their use. As no other convenient reference electrode currently exists for electrochemistry in ionic liquids, potentials are often measured and reported against a quasi-reference electrode (QRE). In the ionic liquid literature, this QRE is typically a silver or platinum wire, which is immersed directly in the electrolyte.
The redox reaction occurring at the surface of the QRE is not known and can vary with wire purity and cleanliness, and therefore the potential of the QRE is likely to vary between labs and experiments. Furthermore, the potential of the QRE can change during the electrochemical experiment due to dissolution, reaction, or polarization, and potential drifts of 10s to 100s of mV throughout experiments are often observed. The potential of the QRE must be reported against a well-defined redox couple (e.g. ferrocene) to allow comparisons of potential to be made between experiments. However, as ferrocene is typically added at the end of the experiment, this one-point calibration of the QRE does not necessarily apply throughout the entire experiment, as the QRE may drift during the measurement.
Currently, the only commercially available REs for ionic liquids or other non-aqueous solvents are based on a silver wire in a tube separated with a porous frit, which contains a fixed concentration of Ag+ ions (typically 10 or 100 mM). This Ag/Ag+ RE exhibits good stability, with a variation of only 1–8 mV between measurements made over the course of a few weeks. However, it must be prepared and used in a glove box and protected from light, as the Ag+ salt is air/moisture and light sensitive. This Ag/Ag+ RE must be prepared frequently due to diffusion of the silver ions out of the electrode, and the photoreactivity of the silver ions.
LLNL has developed a reference electrode that is a great improvement on the widely used silver or platinum wire QRE commonly used in electrochemistry in ionic liquids. This new reference electrode, based on a silver-sulfide coated silver wire, exhibits greatly improved stability over a QRE. The stability of our RE approaches that of the Ag/Ag+ RE, but unlike the Ag/Ag+ RE, the RE reported here is not sensitive to light and can be made and used without a glovebox. Additionally, to the best of our knowledge, the long-term stability of our RE is far superior to any RE reported in the ionic liquid literature.
LLNL's Ag/Ag2S reference electrode can be used in non-aqueous electrochemistry work to provide a stable reference potential throughout multi-day measurements with minimal drift in potential. The Ag/Ag2S reference electrode is a single-junction or double-junction electrode containing the same electrolyte inside and outside of the reference electrode body, thereby minimizing the junction potential. The reference potential is fixed by the silver sulfide coating on the silver wire, which establishes an equilibrium with the filling solution of the reference electrode.
The Ag/Ag2S reference electrode has been found to have minimal interference from air or moisture when assembled and used outside of a glovebox when it is used with air/moisture stable electrolytes.
LLNL's Ag/Ag2S reference electrode provides a major improvement over commonly used quasi-reference electrodes, which have unstable and unknown reference potentials. It is robust and generally maintenance free. Unlike reference electrodes containing a fixed concentration of ions (i.e. Ag+ ions), which are susceptible to potential drift due to leaching or breakdown of the ions, LLNL’s Ag/Ag2S reference electrode relies on a dynamic equilibrium between the stable Ag2S coated Ag wire and the filling solution, thus providing a longer shelf-life and usage lifetime for the reference electrode.
The Ag/Ag2S reference electrode can be used for electrochemistry in any electrolyte, such as ionic liquids, organic solvents, or aqueous solutions. It can be used in an inert atmosphere, or in a normal air atmosphere. The Ag2S coating is very stable after formation, and it is envisioned that this reference electrode can be sold as a kit containing the Ag2S coated Ag wire plus reference electrode tube with a frit, and the customer can fill the reference electrode with their desired electrolyte.
The LLNL Ag/Ag2S reference electrode was initially developed as a 4 mm x 50 mm mini electrode for use in a small volume cell, however, it could be further miniaturized in a capillary tube, or scaled up to a conventional reference electrode size.
LLNL's Ag/Ag2S reference electrode can be used in a wide range of electrolytes, where contamination or precipitation due to the reference electrode filling solution limits the use of other reference electrodes.
Potential applications for LLNL’s Ag/Ag2S reference electrode include:
- Batteries (Li+, Na+, etc.)
- Redox flow batteries
- Fuel cells
- Ionic liquid electrochemistry
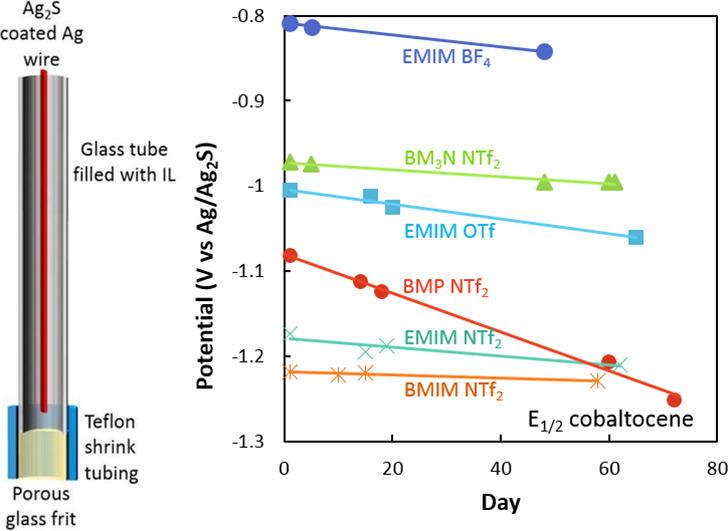
LLNL has filed a patent for the Ag/Ag2S reference electrode. The Ag/Ag2S reference electrode is already in daily use in research at LLNL, where it has been used for the past 12+ months to study electrochemistry in various ionic liquids. The stability of the reference electrode throughout several days of continuous use, and several months of regular laboratory use, have been studied and published in Electrochemistry Communications (Echem Comm. 88 (2018) 105-108). The reference electrode was found to be stable within 2 mV during 4 days of continuous use and was found to drift to more positive potentials by <1 mV per day over the course of 2+ months with regular use in 5 different ionic liquids.