Polyurethanes are major consumer plastic materials, ranking 6th in global plastics production (Delavarde, 2024) with an annual production of 16 million tons (NREL, 2020). Due to their superior mechanical properties, polyurethane polymers are ubiquitous and have been extensively used for a wide range of applications including foams, coatings, adhesives, sealants & elastomers. However, to date, the recycling of polyurethanes, especially with crosslinked networks, is an impractical, low value business due to numerous difficulties associated with reprocessing and/or repurposing these materials. Less than 20% of polyurethanes, mainly polyurethane thermoplastics, are recycled by methods such as rebonding, hydrogenation, and glycolysis. Recycling of polyurethanes is still an unattractive process from an economic standpoint. To date, most recyclable and reprocessable polyurethane systems rely on remolding processes, which generally require high temperatures, metal catalysts, and suffer from a reduction in mechanical properties of the recycled materials relative to the pristine materials. In order to overcome these limitations, many attempts have been undertaken including appropriate selection of catalysts insertion of other dynamic chemistries, and alternative polymerization methods.
LLNL researchers have developed a method which utilizes functional alcohols to depolymerize polyurethane crosslinked networks. The functional alcohols show 5X increase in the depolymerization efficiency compared with current state of art (e.g. methanol, ethylene glycol). The crosslinked polyurethane networks completely depolymerized into a liquid oligomer within 48 hours at ambient temperature. Furthermore, no additional chemical procedures are needed to purify the depolymerized compound.
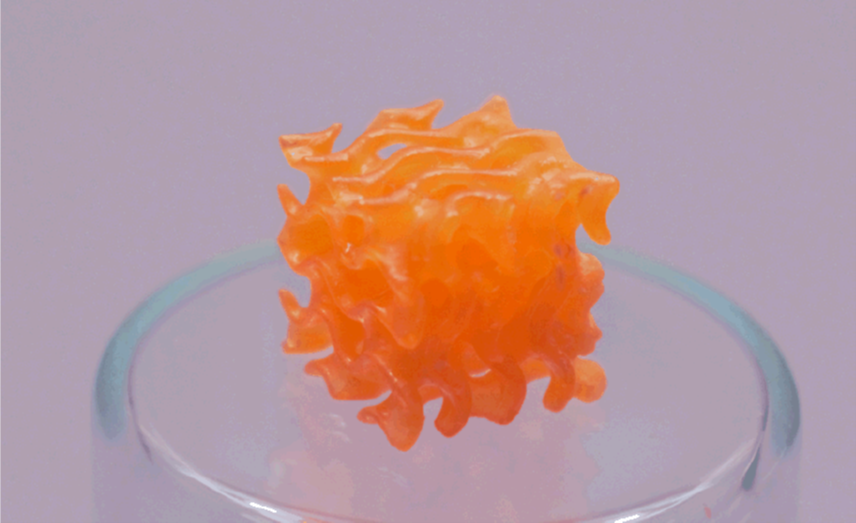
Chemical recycling of the polyurethane with functional alcohols can lead to a diverse materials library through the appropriate re-formulation of the recycled monomers. When mixed with commercially available thiol monomers, the recycled urethane monomers readily undergo fast photopolymerization with thiol functional groups. These resins made from the depolymerized urethane oligomers could then used for 3D printing of structures with high precision with superior mechanical properties compared to conventional photopolymers.
- The crosslinked polyurethane networks completely depolymerized into a liquid oligomer within 48 hours at ambient temperature without needing further purification.
- Functional alcohols show a 5X higher reaction yield compared to state of the art.
- Various types of polyurethanes can undergo this depolymerization process, including rigid foam, flexible foam, coatings, and sprays.
- Depolymerized oligomers can be mixed with commercially available monomers resulting in 3D printable photopolymerizable resins which results in structures having superior mechanical properties.
Polyurethane recycling, 3D printing photoresins
Current stage of technology development: TRL-3
LLNL has filed for patent protection on this invention.