The ion-conductive performance of a polyelectrolyte membrane hinges on its ability to form hydrophilic domains, commonly known as water channels. Ion transport within the membrane predominantly occurs within these ionic highways, where ions traverse through a blend of diffusion, migration, and site hopping mechanisms. These processes are facilitated by anchored ionic sites intricately dispersed along the inner walls of the water channels. The size and winding nature of these channels are shaped by a myriad of factors, including the chemical attributes of the polymer's backbone, ion exchange content, and the length and frequency of ionic functional sidechains. For a given application, the efficacy of polyelectrolyte membranes is contingent upon their proficiency in selectively transporting particular ions while excluding others, as well as any gaseous or liquid byproducts. The selectivity of these materials is closely tied to the dimensions and configuration of the water channels as well as the ion exchange capacity (IEC). Frequently, the dimensions of the water channels are intricately linked to the IEC, making it challenging to manage both selectivity and water uptake (WU) without modifying the IEC. This challenge can result in suboptimal performance, particularly at elevated IEC levels, where there is a disproportionate increase in water absorption and the expansion of hydrophilic channels.
LLNL researchers have developed a method to enhance the performance of polyelectrolyte membranes by using a humidity-controlled crosslinking process which can be applied to precisely adjust the water channels of the membrane. The method allows for membrane performance optimization without changing the fundamental chemistry, thereby enabling the tailoring of the membrane to suit the specific demands of the intended application. The process lends itself to on-demand, purposeful batch variation, based on processing conditions, offering an economical approach for customizing materials on a large scale, particularly for industrial manufacturing.
The novel method utilizes a UV-active small molecule crosslinker previously integrated into the polymer network. The crosslinker must be soluble in the selected solvent used during the evaporation casting process for the final membrane form factor preparation. Once the desired solid form factor is produced, the membranes are then transferred to a controlled humidity chamber. The equilibrated membranes are subsequently exposed to a UV light and cured. During this exposure, effective crosslinking occurs locking in the water channel domain size. Following the curing process, the microstructure is locked into place chemically and can be further processed for utilization and characterization.
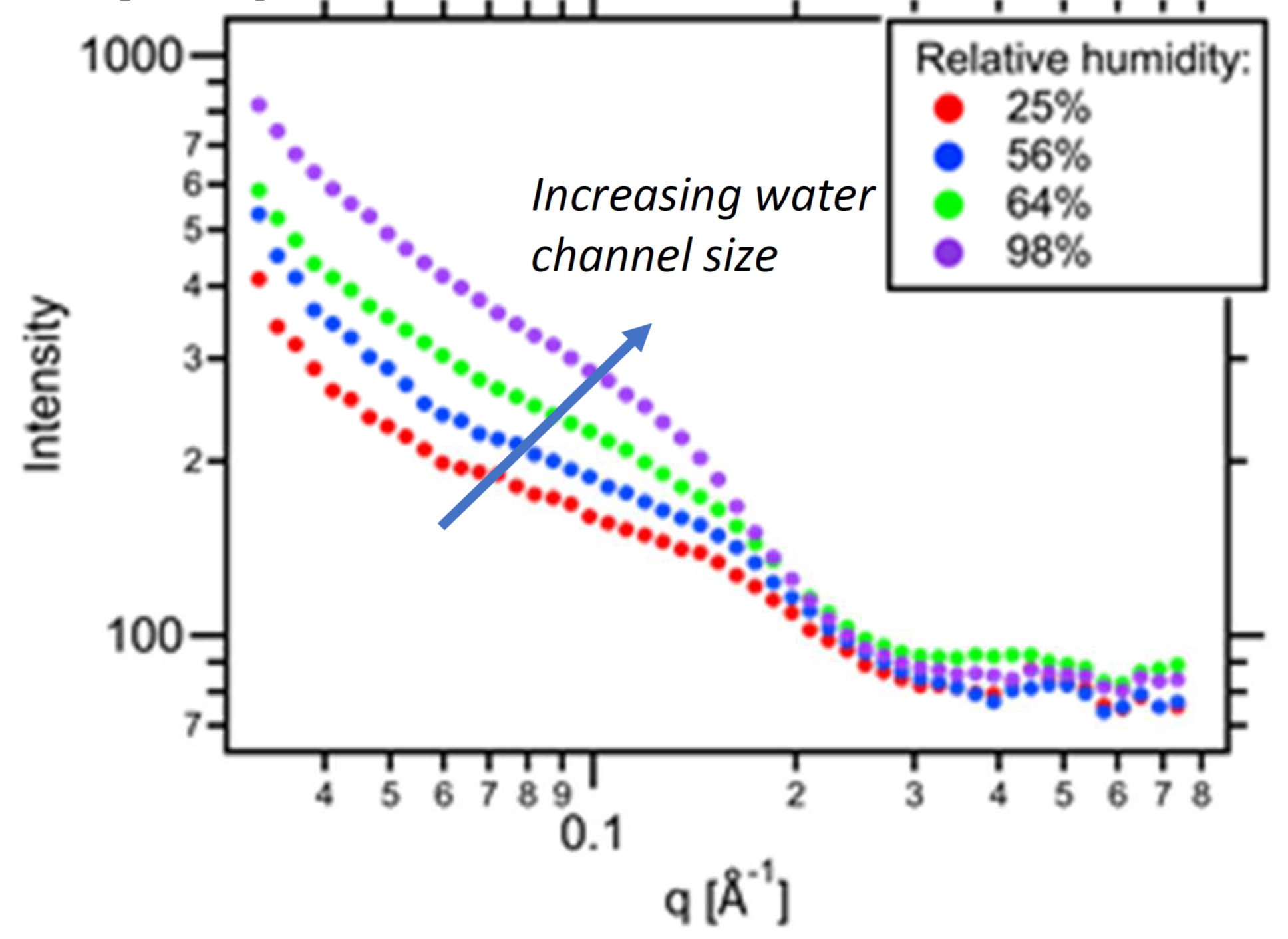
Image Caption: Small-angle X-ray scattering (SAXS) data of crosslinked polyelectrolyte membrane films formed under different equilibrium humidity conditions.
- Method allows for membrane performance optimization without necessitating alterations to the fundamental chemistry.
- Custom tailoring of the membrane to suit the specific demands for a given application.
- Can tune water channel physical dimensions and hence control conductivity and selectivity of the polyelectrolyte membrane.
- Can potentially work with a variety of polymer precursors and small molecule crosslinkers.
CO2 Electrolyzers, proton exchange membranes, solid state battery electrolytes, water and wastewater treatment
Current stage of technology development: TRL 3
LLNL has filed for patent protection on this invention.