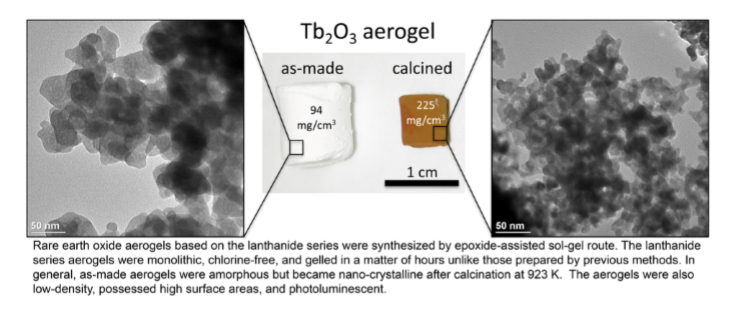
Rare earth oxides (REO) are frequently used as catalysts or catalyst supports due to their high catalytic activity, good thermal stability, and oxidation resistance.1-7 Though there have been many reports on the synthesis of high surface area REO materials,1, 8-10 attempts to make pure, monolithic REO aerogels via the simple epoxide-assisted sol-gel method remain limited.11 Typically, this technique uses a metal chloride as the precursor in an ethanolic solution, which upon the addition of an organic epoxide forms a gel. Unfortunately, the use of the chloride precursor results in formation of a significant oxychloride fraction in the REO aerogels that is extremely difficult to remove and can potentially poison the catalyst.11-14 Alternatively, there have been attempts to use halide-free salts, such as metal nitrates, as the precursor, but in these cases either no gel was formed or the synthesis took an extraordinarily long time to complete (e.g. weeks).8 Therefore, the synthesis of contaminant-free, monolithic REO aerogels via epoxide-assisted sol-gel method remains a significant challenge.
The innovators have modified a epoxide-assisted sol-gel method to produce chlorine-free, monolithic REO aerogels in just a matter of hours. This method was demonstrated for the lanthanide series. An important factor in realizing the sol-gel transition with the nitrate precursor was the addition of a key ingredient and moderate heat.. These alcogels can then be dried and calcined to produce chlorine-free, low-density, high surface area REO aerogels covering the lanthanide elements. Nitrogen porosimetry showed pore sizes in mesopore range (<50 nm) and surface areas up to 150 m2/g for the uncalcined samples. Electron microscopy and XRD analysis show that the aerogels are crystalline after calcination, retaining particle sizes less than 20 nm at temperatures up to 1373K.
- Chlorine-free, monolithic lanthanide oxide aerogels have been a significant challenge via other methods.
- Chlorine-free, low-density REO aerogels were produced by the LLNL method.
- Gelation of the nitrate solution is achieved in a matter of hours.
- Photoluminescence properties of the aerogels are sensitive to the presence of chlorine, with certain components only present in chlorine-free aerogels.
- High surface area and nanocrystalline properties make these aerogels promising candidates for catalysts and catalyst supports.
- Photoluminescent aerogels have potential applications in optical and laser technologies as well as "smart" materials, e.g. insulation that can also serve as a thermometer via phosphor thermometry.
- Catalysts and catalyst supports. There is a potential for doping these materials with transition metals such as nickel or cobalt by similar methods to achieve catalytic properties (e.g. in hydrogenation reactions).
Related research paper: Journal of Sol-Gel Science and Technology https://doi.org/10.1007/s10971-018-4811-y
US Patent No. 11414598 Monolithic rare earth oxide aerogels published 08/16/2022