During CO2 electrolysis, cations need to arrive at the cathode to catalyze the chemical transformations and allow for highly selective carbon dioxide (CO2) reduction. While salts are required to drive efficient electrolysis, they also lead to failure modes. Salts crossing over from the anode to cathode increase the electro-osmotic drag of water which can induce flooding, or if sufficient cations reach the cathode, the concentration can exceed the solubility limit typically in the flow field due to convective drying leading to precipitation and gas blockage.
To overcome the need for salts at the cathode, existing strategies include fixed charge at the cathode surface using polymer electrolytes. Currently, systems that use deionized (DI) water fed systems require a forward bias bipolar membrane. Forward bias bipolar membranes are at a low technology readiness level (TRL) due to the recombination of liquids and gases at the membrane interface leading to mechanical failure and require further research to develop durable systems.
To date, state-of-the-art systems using DI water anolytes can still only achieve up to 80% selectivity for CO at 200 mA cm-2 which is insufficient for commercialization. There is a need for a more selective design to reduce CO2 to CO, a building block for many chemical compounds.
LLNL inventors have developed a gas diffusion electrode (GDE) architecture employing a multi-stack design. The first layer consists of a physical vapor deposited metallic catalyst film which then has a second composite layer deposited on top consisting of a second catalyst material mixed with a polymer electrolytes. The two layers working in tandem allow for near 100% CO selectivity at 200 mA cm-2 for over 40 hours at full cell voltages <3V.
LLNL researchers also have optimized the operating conditions for increasing water transport to the cathode to prevent drying of the system. This allows for a steady-state water flux to the cathode balanced by water consumed at the cathode both electrochemically and through an anion exchange membrane.
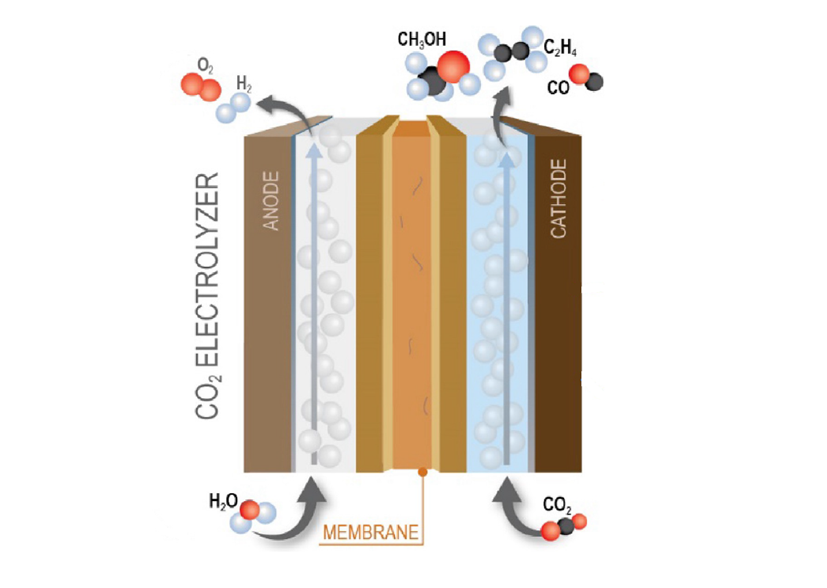
Image Caption: Schematic of a CO2 Electrolyzer
- Allows use of higher TRL anion exchange membranes rather than lower TRL forward bias bipolar membranes.
- Potential ability to customize GDE multi-stack design for specific CO2 reduction target products.
- Demonstrated use under commercially relevant operating conditions for CO2 to CO electrolysis in DI water.
CO2 electrolysis; potentially water electrolysis
Current stage of technology development:
TRL ☐ 0-2 ☒ 3-5 ☐ 5-9
LLNL has filed for patent protection on this invention.