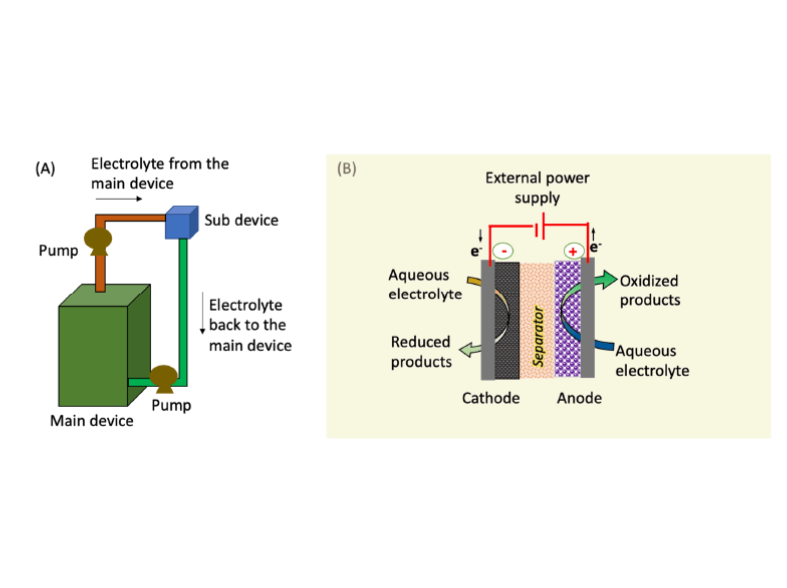
A flow battery (or redox flow battery) is a completely rechargeable electrical energy storage system in which fluids containing the active ingredients are pushed through a cell to promote reduction/oxidation on both sides of an ion-exchange membrane, producing an electrical potential. Viewed as a low-cost alternative for lithium-ion batteries and fuel cells, it has long operational life and low maintenance requirements. The major issue hampering the practical implementation of the redox flow battery is Hydrogen Evolution Reaction (HER) at the negative electrode where plating occurs, which acts as a parasitic competing pathway and causes poor coulombic efficiency (CE).
LLNL researchers has developed an approach to mitigate HER on the ‘plating’ electrode, which uses a sub-device as a rebalancing cell to restore electrolyte properties, including pH, conductivity, and capacity across the main device of the flow battery. This sub-device, which may need to be powered externally, has three major physical components: (1) a cathode electrode, (2) an anode electrode, and (3) a separator to prevent mixing of chemical components (i.e., electrolytes from both sides) and to facilitate selective ion exchange. The cathode electrode can be made of state-of-the-art porous, high surface area carbon-based materials while the anode is composed of porous, high surface area stable water oxidation catalyst material. The separator is an ion selective membrane that is selected based on pH conditions and selective ion transport requirements.
- Value Proposition: Improved durability of flow batteries
- Reduces the rate of HER -> maintains CE
- Lowers system maintenance requirements
- Redox Flow Batteries
- Aqueous media energy storage device
Current stage of technology development: TRL 2-3
LLNL has filed for patent protection on this invention.