Electrochemical devices can generate electrical energy from chemical reactions or can carry out chemical reactions by applying an electric field. These reactors consist of two oppositely charged electrodes (anode and cathode) with a liquid or solid electrolyte in between. This setup leads to fundamental limitations to the system, such as non-uniformities in the electric field, energy losses due to mass transport resistances, and limited volumetric form factors. What is needed is a way to produce previously unachievable configurations to expand the design space for electrochemical devices.
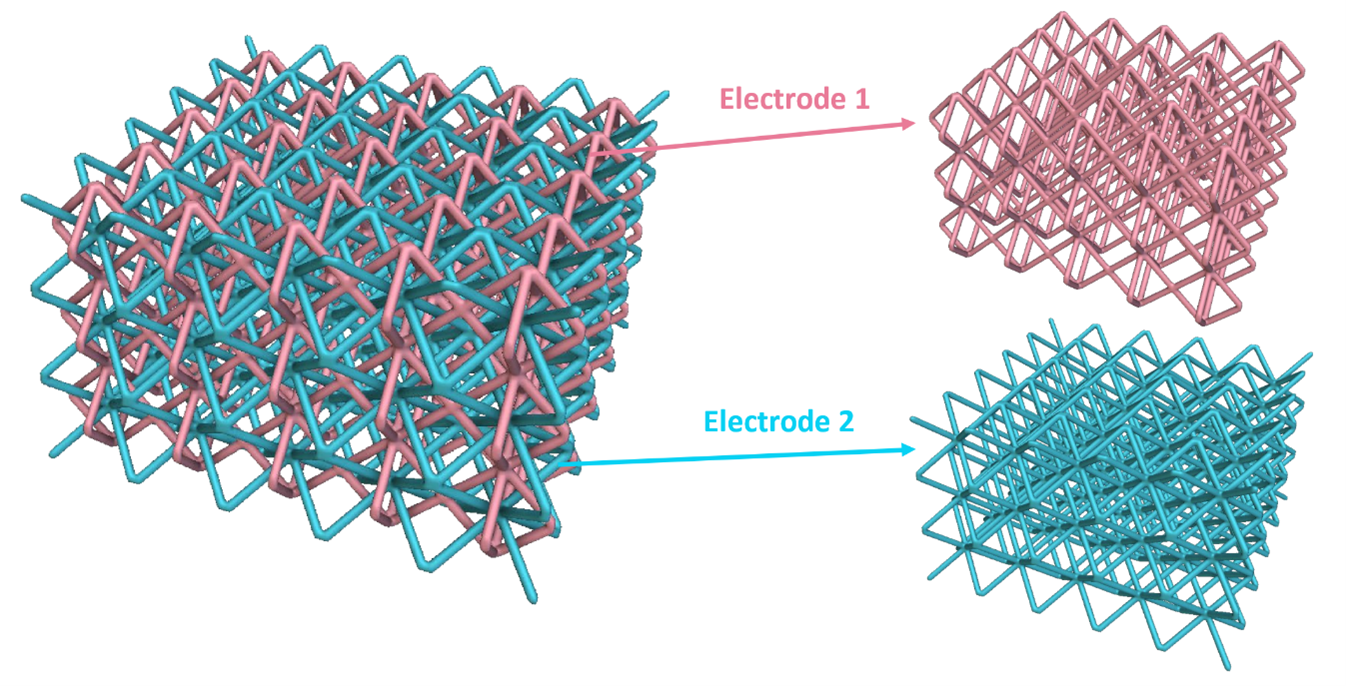
The approach developed by LLNL researchers is to use computer-aided design and advanced manufacturing methods to fabricate two or more continuous electrode structures intertwining in 3D space. This configuration provides improved control electric field uniformity and the ability to carry out multiple electrochemical reactions. This invention utilizes design tools to create architected materials, such as beam-based lattices, triply periodic minimal surfaces (TPMS), or other architectures with repeating structural motifs. The electrode structures are continuous and intertwine in space, while maintaining either a constant or controlled distance between them, and never come in direct contact with each other to prevent short-circuiting. Additive manufacturing can 3D print these interdigitated structures that can then be pyrolyzed to produce an electrically conductive carbon structure. Another approach is to produce the initial structure from a conductive material directly.
- Value Proposition: Ability to fabricate complex designs with improved performance
- Improved control electric field uniformity
- Increased electrochemical reactions within a limited form factor
- Electrochemical devices such as batteries, electrolyzers, fuel cells, CO2 electroreduction reactors, etc.
- Supercapacitors
Current stage of technology development: TRL 4 (Component and/or system validation in laboratory environment)