The current state of the art for water softening is ion exchange in columns packed with functionalized resin. The ion-exchange resins (IERs) work by adsorbing scale forming ions (calcium, magnesium) and replacing them with another ion, sodium, in the softened water. These resins must be periodically regenerated using large amounts of food grade salt, a consumable chemical that must be purchased. Additionally, this water softening method introduces a high salt load into municipal sewer systems and water treatment plants.
An alternative process is to use water desalination technology, such as reverse osmosis (RO). Reverse osmosis uses membranes that allow only water to pass through membranes easily while restricting the passage of contaminants. However, a RO membrane is non-selective; the membrane is capable of removing all the ions present in the water, but not a targeted contaminant. This characteristic reduces the possible efficiency of using RO to treat water for specific trace contaminants such as magnesium and calcium.
A promising method for water softening may be Capacitive DeIonization (CDI), which has the potential to be ion-selective as well as more energy efficient for low-salinity desalination compared to techniques such as RO. Unlike IER or RO methods, CDI removes ions with electric fields, taking advantage of the inherently charged ions, which would naturally be attracted to the charged porous electrodes and thus separating them from the feed water.
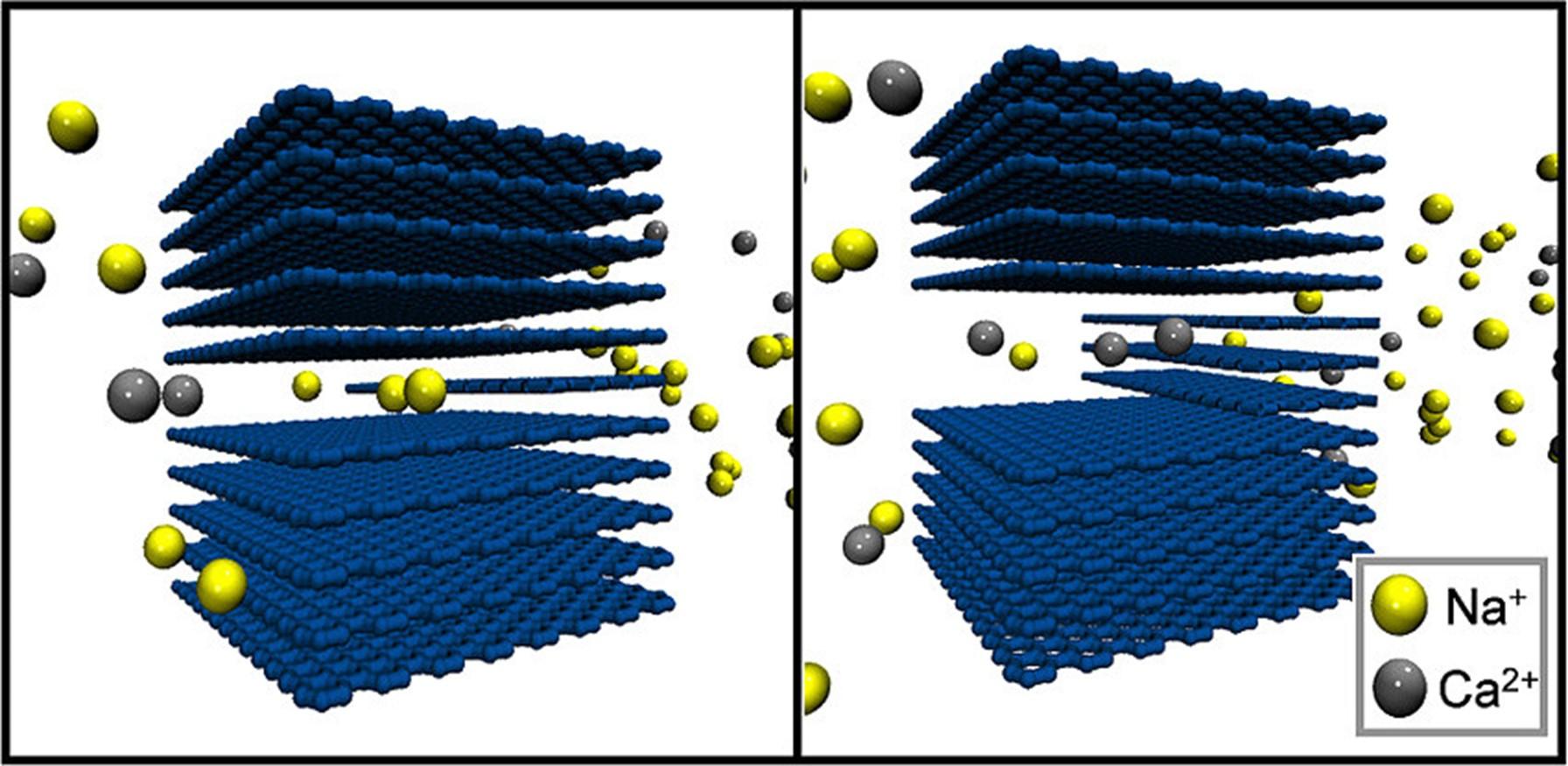
LLNL researchers have developed a novel technique of flow-through electrode capacitive deioinization (FTE-CDI) which can be tailored for selective ion removal from water. It uses porous carbon aerogel materials as capacitive deionization (CDI) electrodes to selectively remove scale forming divalent ions (e.g., magnesium, calcium) from "hard" waters. Through precise control of electrode material synthesis conditions, both a high sorption capacity and a micropore-size distribution is achieved allowing for selective removal of divalent cations. The researchers have been able to demonstrate that their innovative system is capable of achieving an adsorption selectivity of calcium over sodium of more than two-fold. The selectivity can be reversed to favor monovalent ions over divalent ions by changing the electrode synthesis conditions.
Image Caption: Simulation of ion selectivity related to pore size in flow through electrode
M. Cerón, F. Aydin, S. Hawks, D. Oyarzun, C. Loeb, A. Deinhart, C. Zhan, T.A. Pham, M. Stadermann, P. Campbell, Cation Selectivity in Capacitive Deionization: Elucidating the Role of Pore Size, Electrode Potential and Ion Dehydration, ACS Applied Materials & Interfaces 12, 42644 (2020) https://doi.org/10.1021/acsami.0c07903
Water softening with CDI eliminates the need for consumable chemicals and the downstream water treatment requirements.
Unlike the conventional reverse osmosis method, LLNL’s invention enables ion selectivity (e.g., able to favor removal of divalent ions such as magnesium and calcium in the presence of monovalent ions such as sodium). CDI is also potentially more energy efficient when compared to reverse osmosis.
Water softening is performed with inherently low salinity feed water, and thus aligns well with the strengths of CDI.
Water softening: applicable for commercial and residential systems, both of which have large existing markets where barriers to entry are potentially lower as compared to existing desalination/RO.
Current stage of technology development: TRL-3
LLNL has filed for patent protection on this invention.
U.S. Patent Application No. 2021/0061683 Selective Removal Of Scale-Forming Ions For Water Softening published 3/4/2021