Protein manufacturing is a large and growing industry, and provides researchers, clinicians, patients, and consumers the necessary solutions for research, therapy, pharmaceuticals, and nutritional needs. The current industry standard for mass protein fabrication consists of large facilities where genetically-modified cells are cultured to create the desired proteins. These production facilities are slow, expensive as well as labor-intensive as the large fermenting tanks filled with genetically engineered bacterial or mammalian cells, which require constant maintenance and care to ensure the product is properly made and treated. If overlooked, the cultures in the tanks could produce unwanted protein products, resulting in decreased production yield and quality. Additionally, the types of proteins produced in this conventional method are limited due to their inherent instability and/or toxic properties to the cells that create them. Cell-free protein manufacturing can overcome these shortcomings, but it has different disadvantages, e.g. the yield of protein is quite limited because of low density of ribosomes and diffusion and the system is not reusable compared to fermentation systems. If these limitations can be surmounted, a cell-free approach could revolutionize the production of proteins that maximizes yield and portability while minimizing latency and cost.
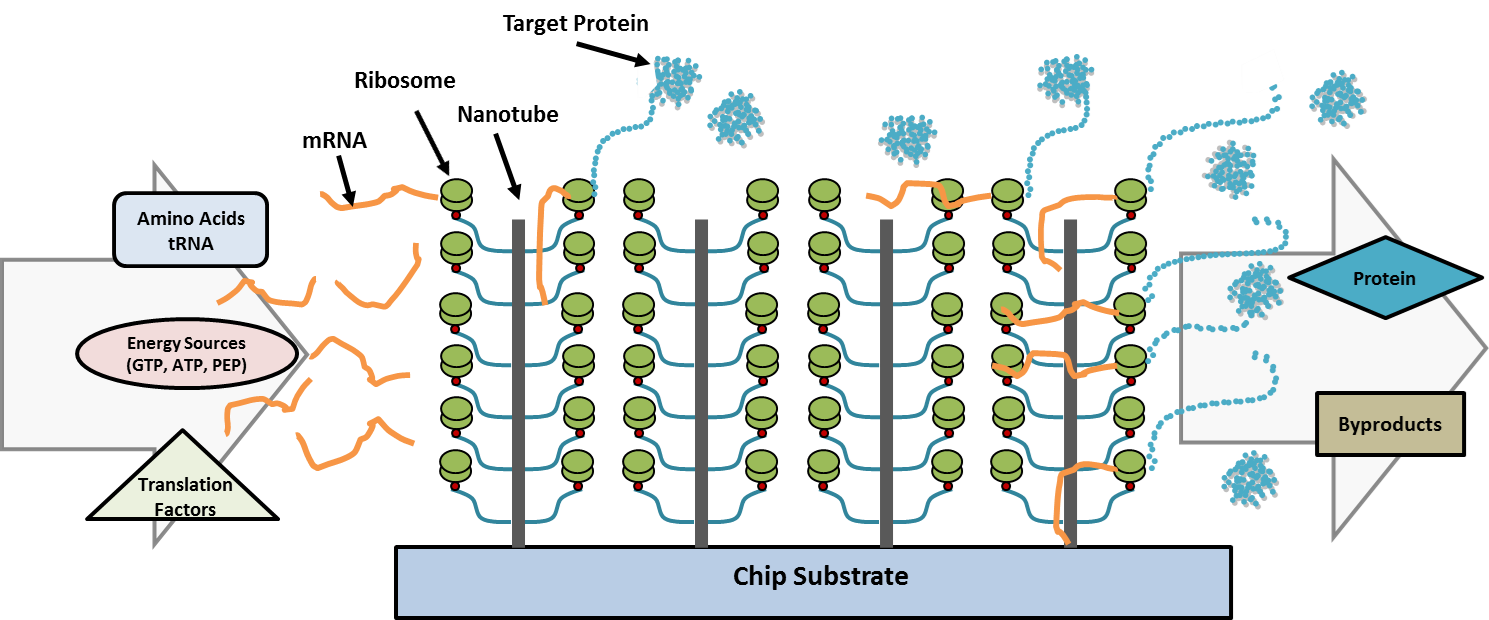
Combining the principles of nanotechnology, cell-free protein synthesis and microfluidics, LLNL researchers have developed a reusable, portable programmable system that can create purified, concentrated protein product in vitro in a microfluidic device containing nucleic acids. Creating high-density arrays for in vitro protein synthesis within a microfluidic device can be achieved by attaching ribosomes to nanotubes that provide additional attachment sites along their length, resulting in a significant increase in ribosomal density on a surface by adding that third dimension.
At the heart of this invention is the microfluidic reaction module, a chamber comprised of billions of nanotubes (2 billion/cm2) containing ribosomes that allows for high surface area (~1,000 times the surface area of flat surface), which maximizes protein translation rate. To rapidly produce virtually any protein, a gene template and components required for in vitro transcription and translation can be injected into this reaction module. After protein synthesis, the translation products and reactants are washed out of the reaction module. The concentration and/or filter module(s) are then used to remove reaction byproducts and unused reactants from the reaction module to produce a purified target protein concentrate.
- Protein configurations are not limited by their instability/toxicity within cells, which is a drawback with industrial manufacturing with largescale fermentation tanks
- Increased production yield and quality via novel ribosomal attachment and precise control over protein translation machinery compared to protein synthesis methods based on biological cells
- Faster and more cost-effective mass production of protein therapeutics
- Portable and on-demand; the technology enables production of a patient-specific protein in small quantity at point-of-care (hospitals) in response to demand.
- Compatible with all known linking chemistries and appropriate for various substrates
- Reusable for producing the same or other proteins since ribosomes are tethered to nanotube arrays
- Enables creation of proteins with novel functions since LLNL’s novel high density protein translation system is an open system
Manufacturing of proteins for
- Therapeutics (including those suitable for personalized medicine approach)
- Biothreat response/recovery
- Research and development (e.g. study of ribosomes in the nanodomain)
Current stage of technology development: TRL-2
US Patent Application No. 2020/0255881 CELL-FREE PROTEIN SYNTHESIS SYSTEMS published 8/13/2020 (https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/20200255881)