A highly controlled microenvironment is required to promote the normal functioning of the central nervous system (CNS). The blood-brain barrier (BBB) is evolved to ensure such a control. It is a biological barrier at the blood to brain interface that effectively separates the brain from the rest of the body, thus able to protect the CNS against pathogens, toxins and xenobiotics. Many CNS pathological conditions are reported to have a defective BBB. These include multiple sclerosis, amyotrophic lateral sclerosis, hypoxic and ischemic insult, Parkinson’s and Alzheimer’s disease, epilepsy, brain tumors, glaucoma, and lysosomal storage diseases. There is an unmet need for an effective in vitro model of the neurovascular unit (NVU) that can both recapitulate and monitor physiologically relevant changes to both the BBB and brain function. The NVU would provide a powerful platform to study neurological diseases, existing and emerging chemical and biological threats, and for development and evaluation of new brain therapeutics.
LLNL inventors have developed a proprietary concept and a prototype NVU device that provides an effective in vitro model of the BBB to discover, develop and screen drug candidates for their ability to cross the BBB. The device could also be useful in studying neurological disorders with defects in BBB to understand the mechanism underlying the pathology of the disease that might reveal modalities of therapeutic intervention. The NVU device provides a means of culturing or co-culturing cells from the CNS in 3D and studying responses to electrical and chemical stimuli in a physiologically relevant manner with features that mimic BBB.
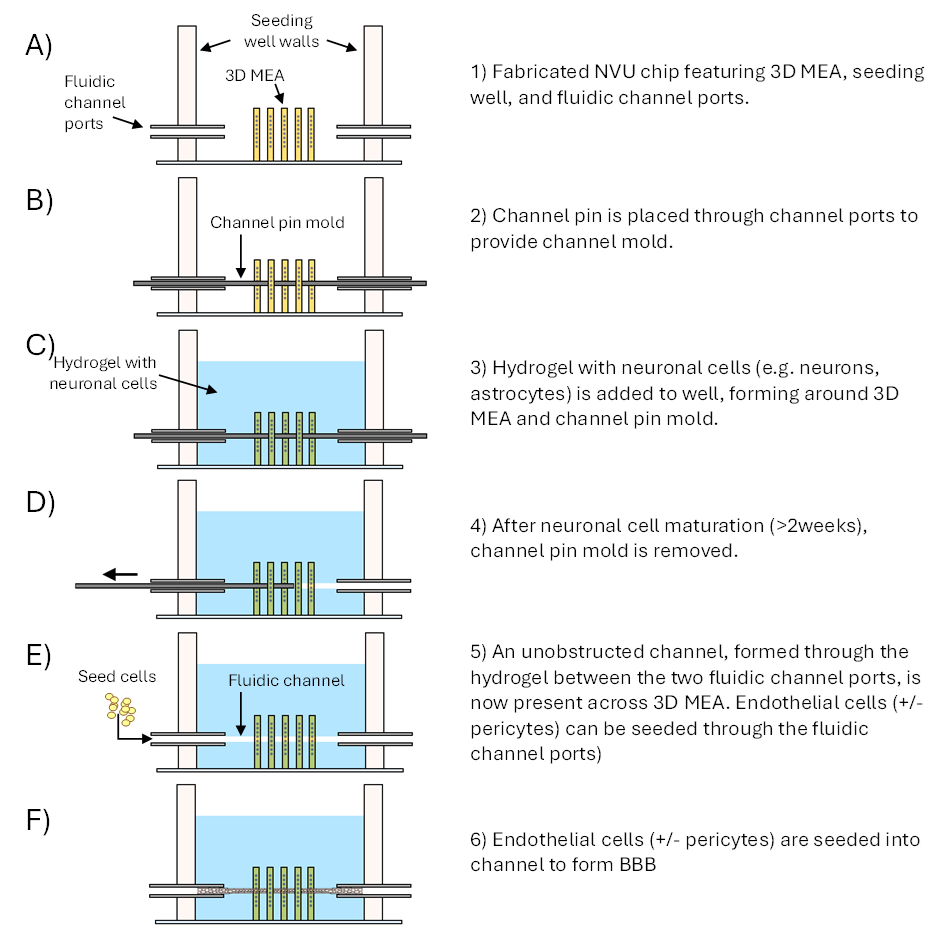
Image Caption: Schematic outlining the formation of the NVU within the engineered system
The NVU device has specific advantages as it:
- supports neuronal co-cultures in a hydrogel directly in contact with endothelial cells deposited on the inner surface of a microfluidic channel formed through the hydrogel
- allows static or continuous flow through the vascular channel
- incorporates 3D multielectrode arrays (MEAs) to non-invasively interrogate the neuronal coculture, and
- interfaces with commercial electrophysiology instrumentation.
The device has broad applications in the area of research and testing of drugs using 3D cell culture, particularly in neuroscience area and specifically for testing the ability of a drug candidate to cross the blood-brain barrier. Additional areas include neuronal communication in 3D, blood brain barrier dysfunction, drug development, drug permeability, countermeasure validation, neuroinflammation, pathogen infection, chemical exposure, response of electroactive cells in 3D, disease research (Alzheimer's, Parkinson's, epilepsy, TBI, ALS etc.), recording/stimulation of spheroids/organoids, and microfluidic delivery of chemicals in 3D.
Current stage of technology development: TRL 2
LLNL has filed for patent protection on this invention.