Chimeric Antigen Receptor (CAR) therapies modify the patient’s own T lymphocytes (autologous) ex vivo and then inject them back into the patient’s body to kill cancer cells. CAR-T cells express hybrid (chimeric) molecules that contain an antibody-binding fragment (scFV) derived from an antibody and intracellular signaling domain(s) from the T-cell receptor. CAR-T cells then bind to specific antigens on the surface of cancer cells resulting in T cell activation and tumor killing.
Typically, a virus is used to introduce the gene for CAR into the T-cells then the cells are activated and amplified ex vivo. The disadvantages of using viral vectors for the delivery of the CAR gene are (i) time-consuming manufacturing that takes 3+ weeks at a GMP facility, (ii) cost (>$300K), and (iii) risk of cancer arising from viral integration in host cells.
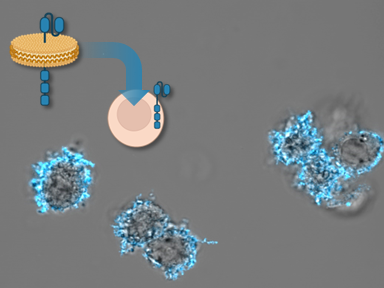
Rather than using genes carried by viruses, LLNL researchers have developed an alternative approach of delivering CAR to T-cells in form of proteins that are carried on the surface of nanolipoprotein (NLP) particles. NLPs are naturally occurring molecules that serve as structural mimics of cell membranes. They can self-assemble and provide a structure or platform for connecting other molecules.
CAR proteins integrated into NLPs show high solubility and appropriate binding to their ligand. Culturing T cells with NLPs results in transfer of CAR proteins into human T cells with minimal toxicity, resulting in a population of CAR expressing T cells with no genetic alterations.
For more information regarding the patented method and systems for producing NLP particles, which is also available for licensing from the Lab, visit Cell-Free Assembly of Nanolipoprotein Particles (IL-11841).
Image Caption: Nanolipoproteins containing CAR proteins can be used to engineer human T cells. This rapid method for protein delivery could represent a new method to manufacture cancer therapies.
• Faster manufacturing of engineered autologous T-cells for cancer immunotherapy
• High efficiency of protein delivery as compared to viral vector-based transfection method
• No risk of genomic DNA integration and therefore cancer
• Rapid response to evolving virus pathogens in controlling pandemics
• Design, and biochemical study of CAR proteins
• CAR-T cell biology
• Immunotherapy of cancer
Current stage of technology development: TRL2
LLNL has filed for patent protection on this invention.