Radioisotopes of mercury - 197gHg (half-life of 64.1 hr) and 197m,gHg (half-life of 23.8 hr) - emit gamma-rays and are suitable for imaging by single-photon emission computed tomography (SPECT) and positron emission spectroscopy (PET) as well as for therapeutic applications. These radioisotopes with low specific activities (produced from neutron reactions on natural stable isotopes of Hg in nuclear reactors) were used for imaging in the 1950s and 1960s. However, they fell out of favor because of their high toxicity associated with stable isoform of Hg present in the preparation.
The production of radiopharmaceuticals in cyclotrons by charged particle reactions of 197Au, 194Pt or 195Pt allows for no-carrier-added (NCA) radionuclides to be produced. This has led to a renewed interest in 197m,gHg and 197gHg for imaging and therapy since they can be produced in large quantities without the toxic stable isotope of Hg present. Previous work on separations of NCA 197m,gHg and 197gHg from Au foils has mostly been focused on distillation of the metal, sublimation of HgCl2, or liquid-liquid extractions. However, these methods suffer from several drawbacks such as product losses, low efficiency, and difficult post processing of purified material
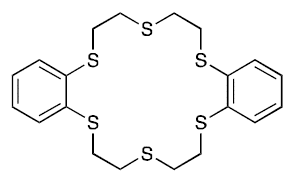
The method described in a pending patent application uses a novel thiacrown (dibenzohexathia-18-crown-6) for efficient extraction of 197m,gHg and 197gHg from irradiated Pt target foils. The separation of 197m,gHg and 197gHg from Pt foils using this novel thiacrown was found to be highly specific. No detectable amount of the Pt foil was seen in the organic phase of the initial Hg extraction. The final recovery of 197m,gHg and 197gHg was 99.8%. The extraction process is not only efficient but also simple and extremely fast. It is possible to perform the entire extraction within one hour. Moreover, this method can be performed similarly on 197m,gHg and 197gHg produced from proton irradiations of Au with the same experimental procedure. Furthermore, there is no presence of stable isotope of Hg since the reaction did not involve stable Hg as a starting material. The toxicity of Hg radioisotopes is very low because they are used at very low concentration for imaging and therapeutics.
Despotopulos, J. D., Kmak, K. N. 2021. "Rapid isolation of 197m,g-Hg from proton irradiated Au foils," J. Radioanal. Nucl. Chem. 327: 1349-1354. https://doi.org/10.1007/s10967-021-07609-y
Despotopulos, J.D., Kmak, K.N. & Shaughnessy, D.A. Production and isolation of 197m,gHg. J Radioanal Nucl Chem 317, 985–989 (2018). https://doi.org/10.1007/s10967-018-5927-9
Despotopulos, J.D., Kmak, K.N., Gharibyan, N. et al. Production and isolation of homologs of flerovium and element 115 at the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry. J Radioanal Nucl Chem 308, 567–572 (2016). https://doi.org/10.1007/s10967-015-4500-z
LLNL’s novel method allows for rapid separation of 197m,gHg and 197gHg from either a Pt or Au foil which has been irradiated using an accelerator. The method uses a novel thiacrown ether for the extraction, and is simple, fast and highly efficient. Existing methods suffer from several drawbacks such as product losses, low efficiency, and difficult post-processing of purified material. The novel thiacrown can be prepared inexpensively; the radiopharmaceuticals 197m,gHg and 197gHg can be made locally at a hospital using a medical cyclotron. Mercury radioisotopes have applications in imaging (particularly brain) as well as cancer therapeutics. Additionally, the method is amenable to scale-up and has environmental application in the recovery of mercury from liquid stream.
Medical isotopes for imaging (e.g. brain) and therapeutics (e.g. for cancer), mercury recovery from liquid stream (environmental applications).
Current stage of technology development: TRL-2
U.S. Patent Application Publication No. 2021/0287822 Methods of isolating radioactive mercury and uses thereof published 9/16/2021
U.S. Patent No. 11587694 Methods of isolating radioactive mercury and uses thereof published 2/21/2023